Traumeel Prescribing Information
Package insert / product label
Dosage form: injection
On This Page
Traumeel Description
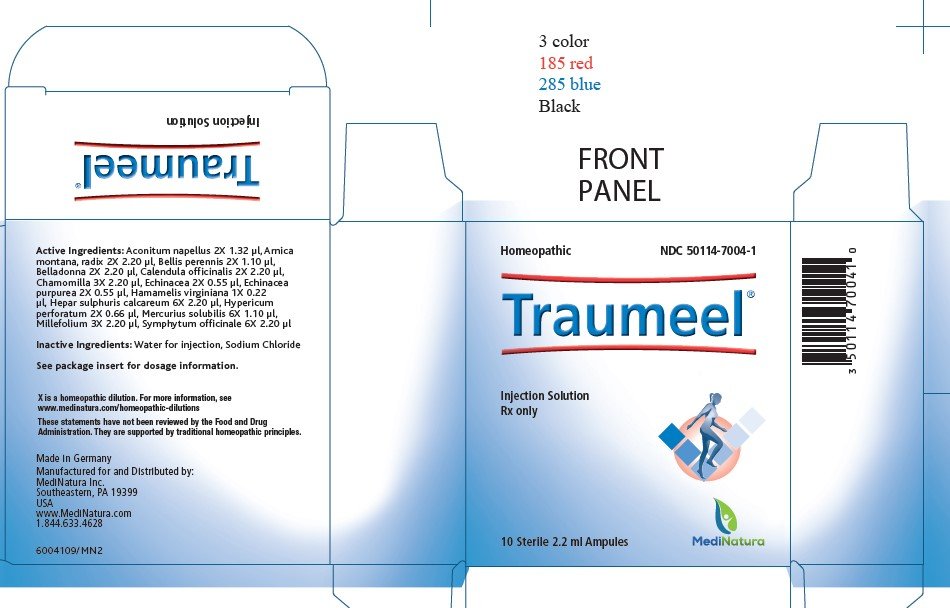
Active Ingredients:
Ingredient name Potency Quantity Final dilution
Aconitum napellus 2X 1.32 μl 5.22X
Arnica montana, radix 2X 2.20 μl 5.00X
Bellis perennis 2X 1.10 μl 5.30X
Belladonna 2X 2.20 μl 5.00X
Calendula officinalis 2X 2.20 μl 5.00X
Chamomilla 3X 2.20 μl 6.00X
Echinacea 2X 0.55 μl 5.60X
Echinacea purpurea 2X 0.55 μl 5.60X
Hamamelis virginiana 1X 0.22 μl 5.00X
Hepar sulphuris calcareum 6X 2.20 μl 9.00X
Hypericum perforatum 2X 0.66 μl 5.52X
Mercurius solubilis 6X 1.10 μl 9.30X
Millefolium 3X 2.20 μl 6.00X
Symphytum officinale 6X 2.20 μl 9.00X
Indications and Usage for Traumeel
Treatment of injuries and various conditions of the musculoskeletal system.
• Traumeel® Injection Solution is a homeopathic drug product indicated for the treatment of injuries, inflammatory and degenerative conditions of the musculoskeletal system and for the relief of associated symptoms such as pain
Co-administration Therapy with Zeel® Injection Solution for the treatment of inflammatory and degenerative conditions of the musculoskeletal system.
• Traumeel ® Injection Solution is a homeopathic drug product indicated, in combination with Zeel® Injection Solution, for the treatment of inflammatory and degenerative conditions of the musculoskeletal system, such as arthrosis/osteoarthritis and/or rheumatic joint diseases, and for the relief of symptoms including pain, swelling, and joint stiffness.
Traumeel Dosage and Administration
General Considerations
- The dosage schedules listed below can be used as a general guide for the administration of Traumeel® Injection Solution.
-
If co-administration with a local anesthetic is desired, Traumeel® Injection Solution may be mixed with lidocaine or similar agents at the discretion of the physician.
- Traumeel® Injection Solution may be administered s.c., i.d., i.m., i.a. or i.v.
- The interval between injections is left to the discretion of the HCP, but should not exceed 1 ampule in 24 hours.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Draw up the contents of the ampule into the syringe. Discard half or one third of the contents, depending on the required dosage, before administering.
-
Only licensed practitioners with sufficient expertise in injecting drugs, including the respective route of
administration, should administer the product.
Standard Dosage - for the treatment of injuries, inflammatory and degenerative conditions of the musculoskeletal system and for the relief of associated symptoms such as pain.
Adults and children 12 years and older:
1 ampule 1 to 3 times per 7 days
Children 6 to 11 years:
2/3 of an ampule 1 to 3 times per 7 days
Children 2 to 5 years:
1/2 ampule 1 to 3 times per 7 days
Acute Dosage - for the treatment of injuries, inflammatory and degenerative conditions of the musculoskeletal system and for the relief of associated symptoms such as pain.
Adults and children 12 years and older:
1 ampule daily, and then continue with standard dosage
Children 6 to 11 years:
2/3 of an ampule daily, and then continue with standard dosage
Children 2 to 5 years:
1/2 ampule daily, and then continue with standard dosage
Co-administration therapy with Zeel® Injection Solution - for the treatment of inflammatory and degenerative conditions of the musculoskeletal system, such as arthrosis/osteoarthritis and/or rheumatic joint diseases, and for the relief of symptoms including pain, swelling, and joint stiffness.
- In the treatment of musculoskeletal conditions, if co-administration with another homeopathic medicinal product is desired, Traumeel® Injection Solution may be mixed in a ratio of 1:1 with Zeel® Injection Solution.
- For convenience, the daily dose of Traumeel® Injection Solution may be administered at the same time as a Zeel® Injection Solution, according to the dosing recommendations for each medication.
Contraindications
- Traumeel® Injection Solution is contraindicated in patients with known hypersensitivity to Traumeel® or any of its ingredients.
Adverse Reactions/Side Effects
Post-marketing Experience
• The following adverse events have been identified during post-marketing use of Traumeel® Injection Solution. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
• Adverse event rates observed in Monotherapy use of Traumeel® Injection Solution: Allergic (hypersensitivity) reactions (e.g. skin allergies, redness/swelling at the injection site, even up to anaphylaxis) may occur in isolated cases.
• Adverse event rates observed in the Monotherapy use of Zeel® Injection Solution: Allergic (hypersensitivity) skin reactions may occur in isolated cases.
•To report SUSPECTED ADVERSE REACTIONS, contact MediNatura at 1.844.633.4628 or info@medinatura.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Overdosage
No negative effects of an overdose have been reported and none are expected due to the homeopathic dilutions.
Traumeel - Clinical Pharmacology
Mechanism of Action
The exact mechanism of Traumeel® Injection Solution is not fully understood.
Pharmacodynamics
Not applicable for homeopathic medicinal products.
| TRAUMEEL
arnica montana root, atropa belladonna, calendula officinalis flowering top, matricaria recutita, achillea millefolium, calcium sulfide, comfrey root, aconitum napellus, bellis perennis, mercurius solubilis, hypericum perforatum, echinacea, unspecified, echinacea purpurea and hamamelis virginiana root bark/stem bark injection |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Medinatura (102783016) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biologische Heilmittel Heel | 315635359 | manufacture(50114-7004) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hameln Pharma GmbH | 315869123 | manufacture(50114-7004) | |